Description
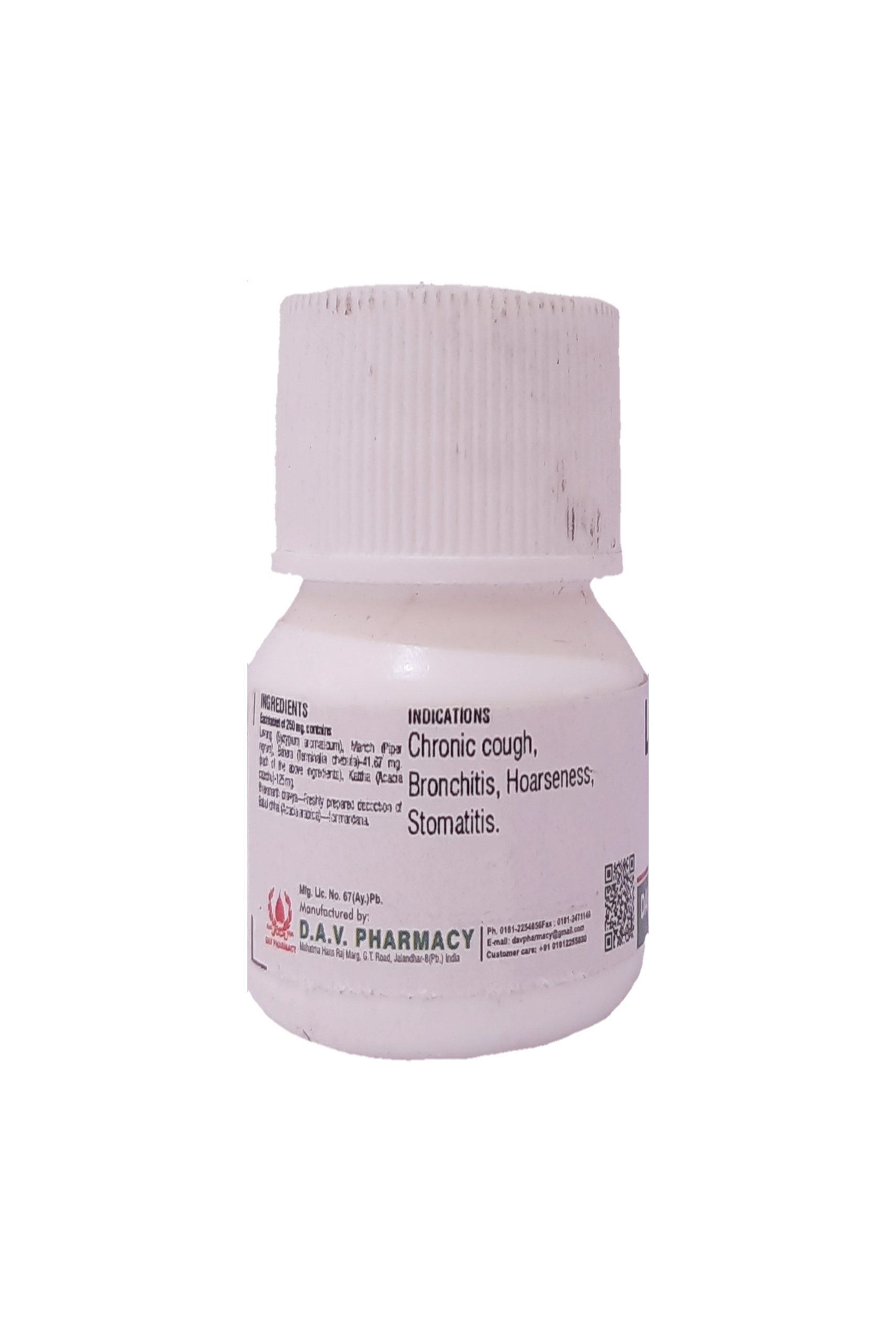
NAME OF MEDICINE :- KHADIRADI VATI
CLASSICAL REFERENCE :- Rastantarasara-Mukharogadhikara.
FORMULATION COMPOSITION :-
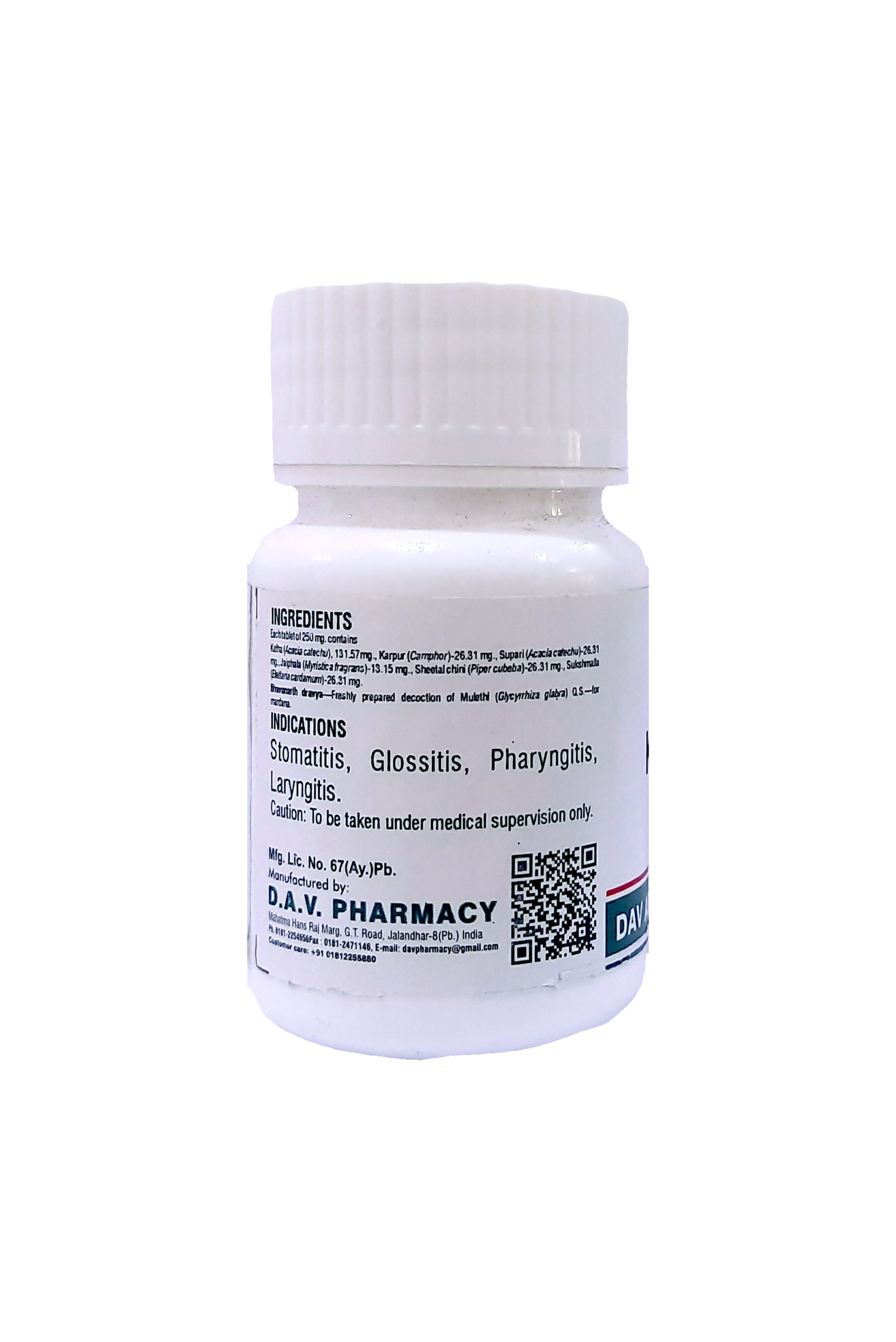
Each tablet of 250 mg. contains
Katha (Acacia catechu), 131.57mg., Karpur (Camphor)-26.31 mg., Supari (Areca catechu)-26.31 mg., Jaiphala (Myristica fragrans)-13.15 mg., Sheetal chini (Piper cubeba)-26.31 mg., Sukshmaila (Elettaria cardamum)-26.31 mg.
Bhavanarth dravya—Freshly prepared decoction of Mulethi (Glycyrrhiza glabra) Q.S.—for mardana.
DOSE :-
1 to 2 tablets or as directed by the physician.
THERAPEUTIC USE :–
Useful in stomatitis, ulcerative stomatitis, dryness of mouth, glossitis, pharangitis & hoarseness of voice. Can be consumed orally or can be used for chewable purpose in all the above mentioned diseases.
ANUPAN :-
To be taken twice or thrice in a day or can be chewed or as directed by the physician.