Description
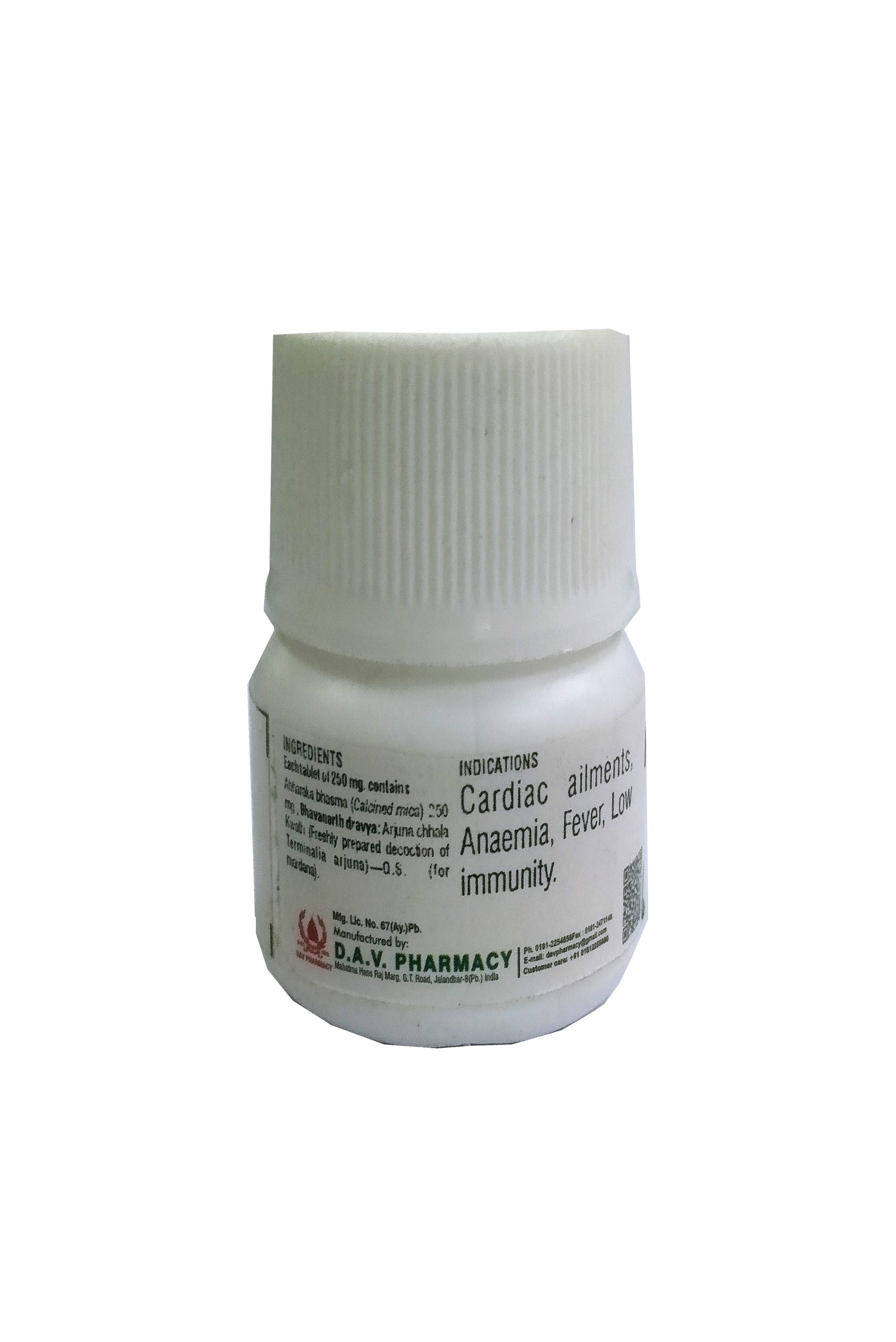
NAME OF MEDICINE :- CHITRAKADI VATI
CLASSICAL REFERENCE :- Rasendrasarasangraha-Ajirnarogadhikara.
FORMULATION COMPOSITION :-
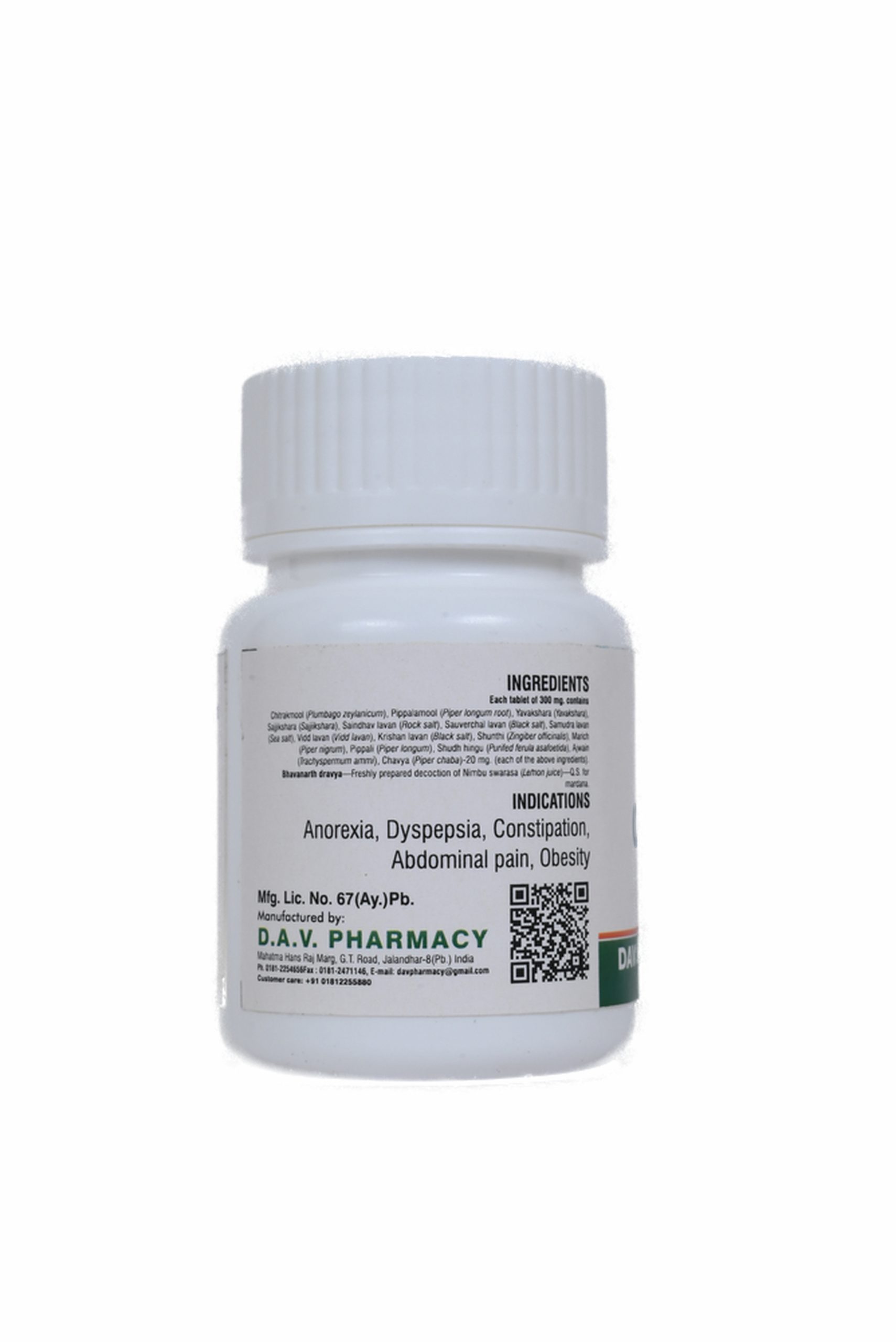
Each tablet of 300 mg. contains
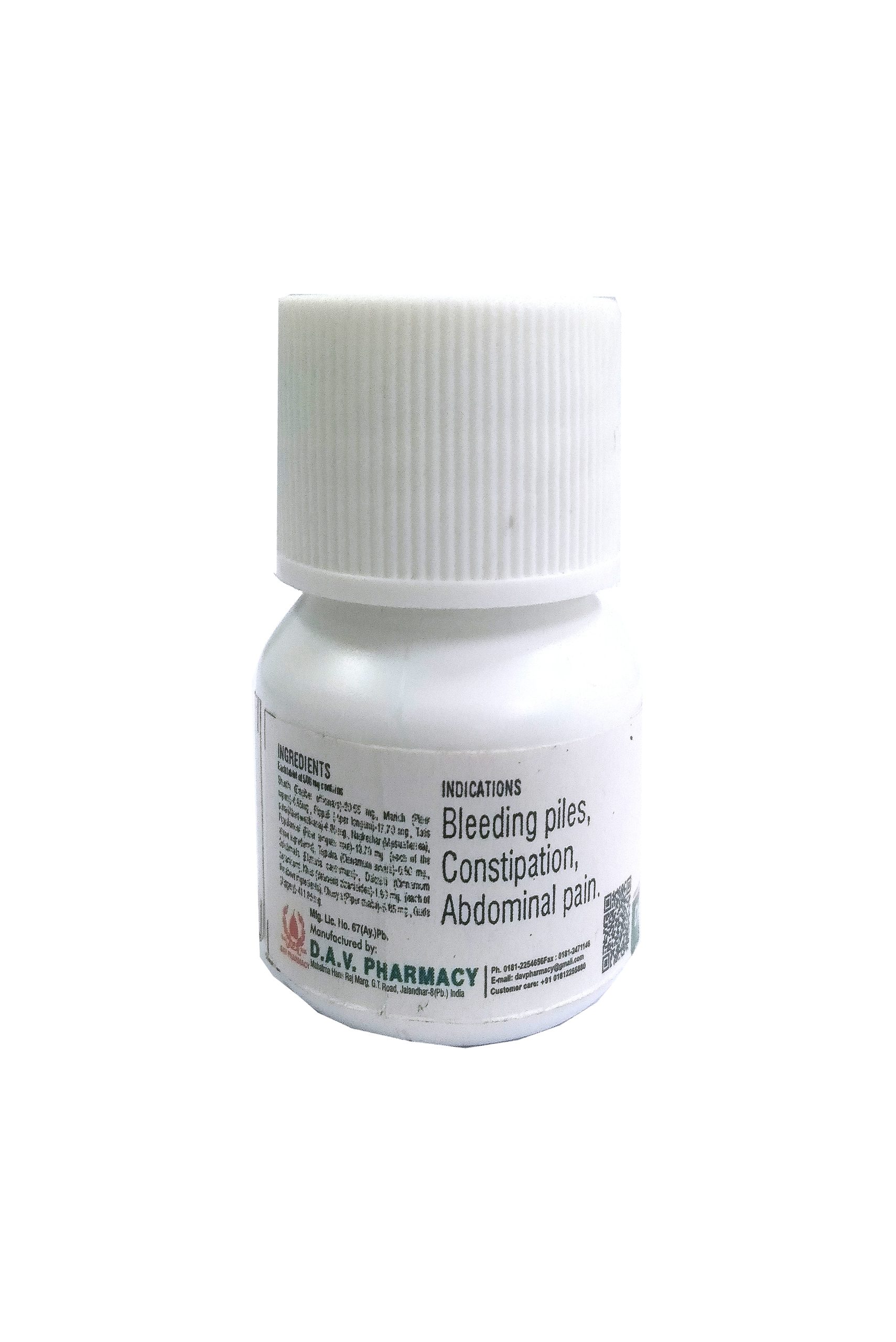
Chitrakmool (Plumbago zeylanicum), Pippalamool (Piper longum root), Yavakshara (Yavakshara), Sajjikshara (Sajjikshara), Saindhav lavan (Rock salt), Sauverchal lavan (Black salt), Samudra lavan (Sea salt), Vidd lavan (Vidd lavan), Krishan lavan (Black salt), Shunthi (Zingiber officinalis), Marich (Piper nigrum), Pippali (Piper longum), Shudh hingu (Purifed ferula asafoetida), Ajwain (Trachyspermum ammi), Chavya (Piper chaba)-20 mg. (each of the above ingredients).
Bhavanarth dravya—Freshly prepared decoction of Nimbu swarasa (Lemon juice)—Q.S. for mardana.
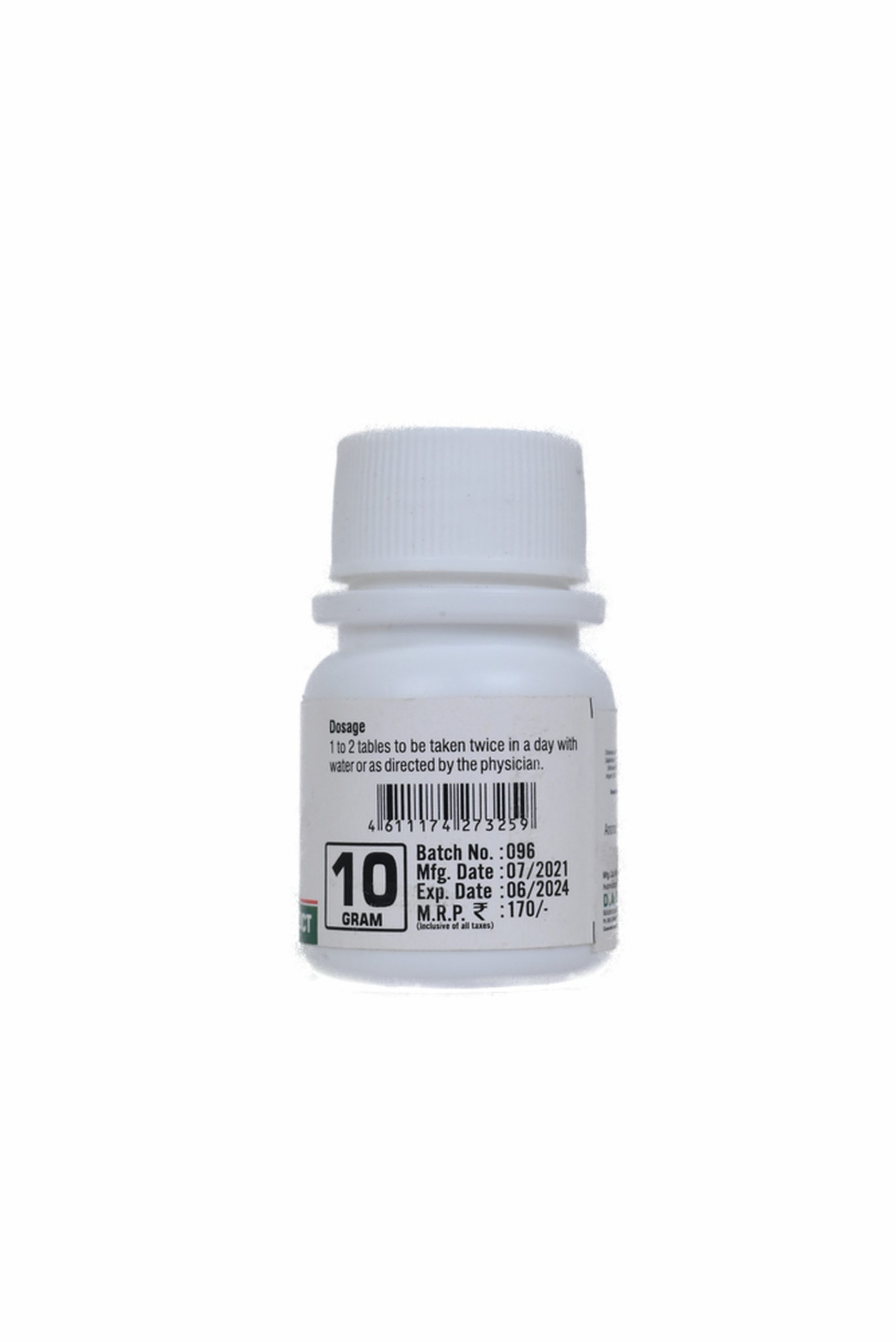
DOSE :-
1 to 2 tablets or as directed by the physician.
THERAPEUTIC USE :–
Useful in anorexia, dyspepsia, digestive impairment, sprue, abdominal pain, constipation, oligouria etc.
ANUPAN :-
To be taken twice in a day with water or as directed by the physician.