Description
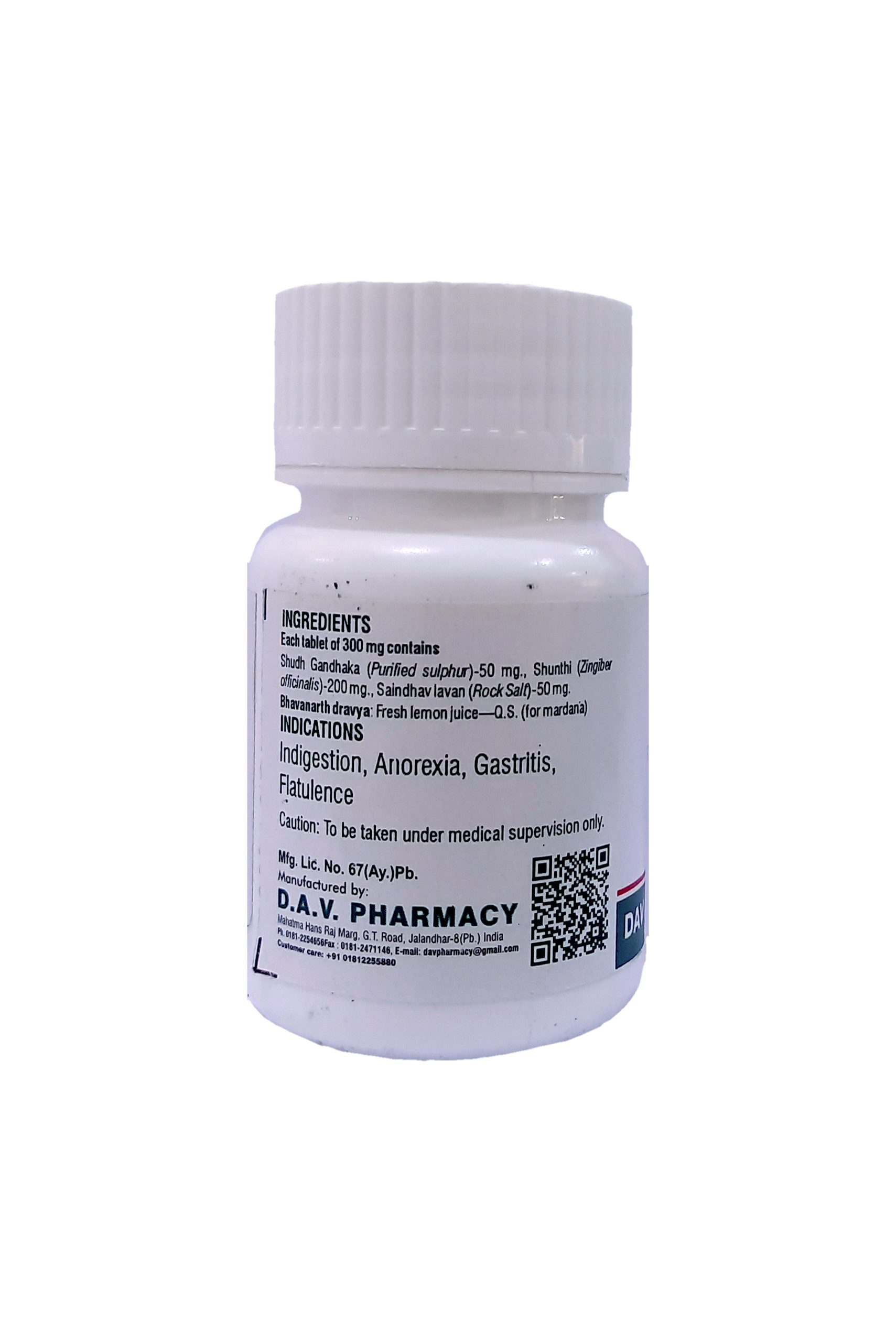
NAME OF MEDICINE :- GANDHAK VATI
CLASSICAL REFERENCE :- Rasaratanasamuchaya-Ajirnarogadhikara
FORMULATION COMPOSITION :-
Each tablet of 300 mg. contains
Shudh Gandhaka (Purified sulphur)-50 mg., Shunthi (Zingiber officinalis)-200 mg., Saindhav lavan (Rock Salt)-50 mg.
Bhavanarth dravya: Fresh lemon juice—Q.S. (for mardana)
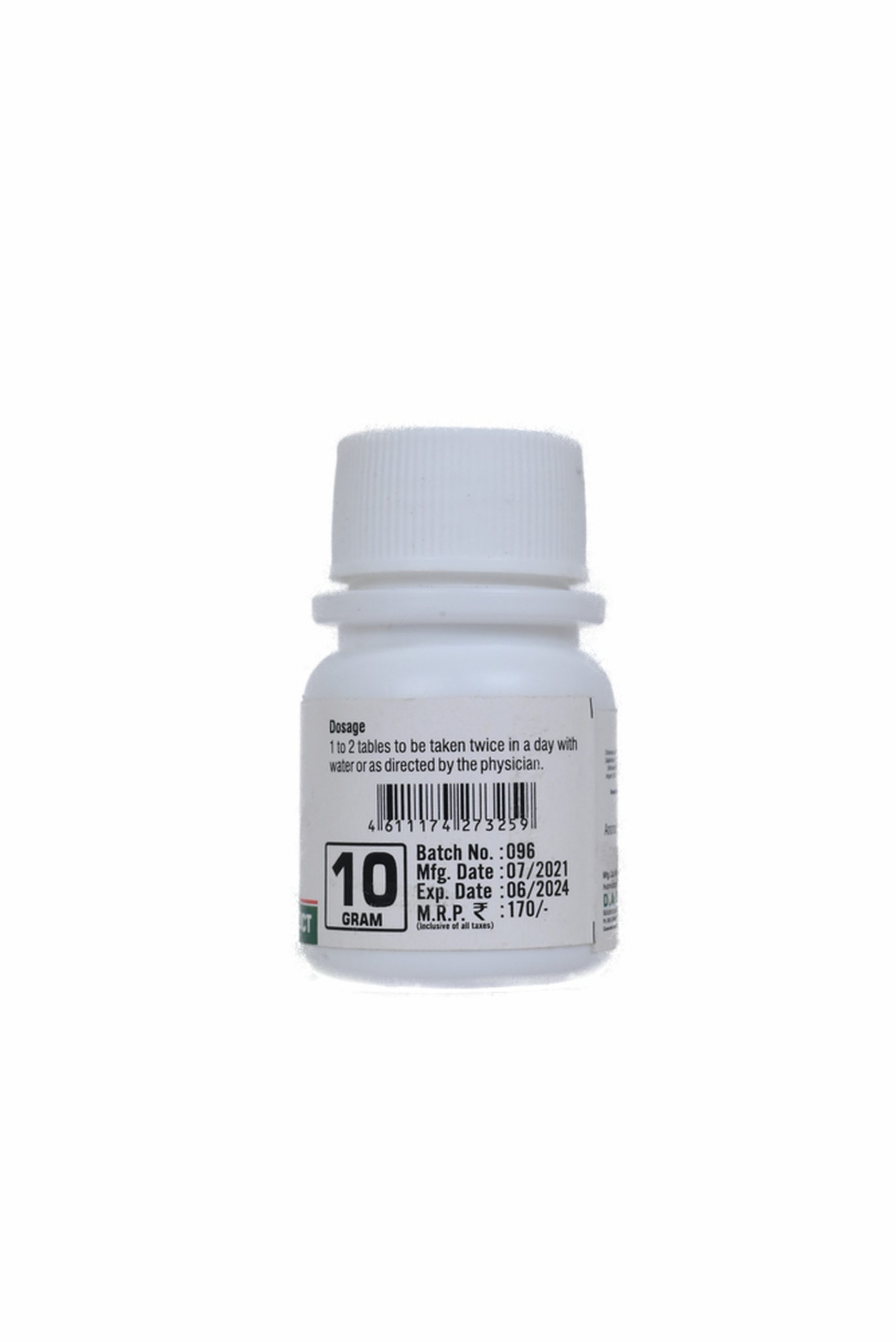
DOSE :-
1 to 2 tablets or as directed by the physician.
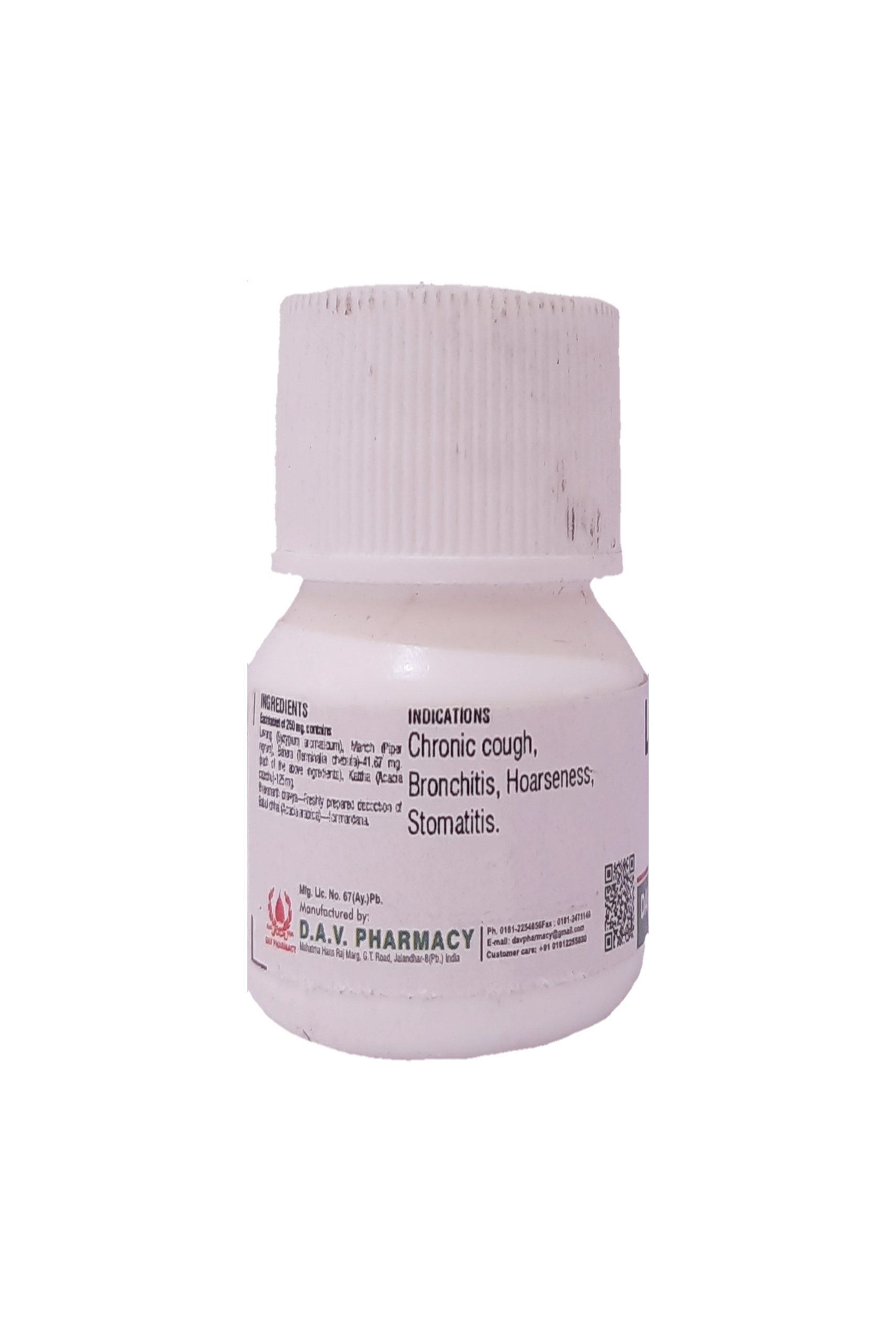
THERAPEUTIC USE :–
Carminative and digestive. Famous as tasty digestive tablet. Useful in anorexia, dyspepsia, flatulence, abdominal pain, constipation & hyper acidity.
ANUPAN :-
To be taken twice or thrice in a day with luke warm water or as directed by the physician.